
If you’ve yet to be asked about prescribing medicinal cannabis, there’s a good chance your patients will be requesting information in the coming months.
Data released by the Ministry of Health under the Official Information Act shows that the number of packs of medicinal cannabis prescribed and supplied in New Zealand has grown at an average rate of 250% annually. In the year to 30 June 2021, 31,000 packs of medicinal cannabis were supplied in New Zealand compared with just 2,000 in 2018.
Studies have shown that many healthcare practitioners considered themselves to be lacking knowledge surrounding the pharmacology, pharmacokinetics and pharmacodynamics of medical cannabis. But with more product registrations from Medsafe in recent months, healthcare practitioners need to ensure they are adequately equipped to provide clinical advice and oversight in the safe management and dispensing of medical cannabis.
The legalities of medicinal cannabis in New Zealand.

Many NZ pharmacists believe that patients need to be referred to a specialist to receive a prescription for medicinal cannabis in New Zealand but this is incorrect.
Since the Medicinal Cannabis Scheme came into effect on 1 April 2020, GPs have legally been able to prescribe CBD, THC or a combination of these products on the proviso they have been manufactured according to current Good Manufacturing Practices (cGMP) and conform to strict quality standards.
However, as these medicinal cannabis products are currently unapproved by MedSafe, they will not be funded by Pharmac, and will be needed to be prescribed under Section 29 of the Medicines Act.
The workings of the endocannabinoid system
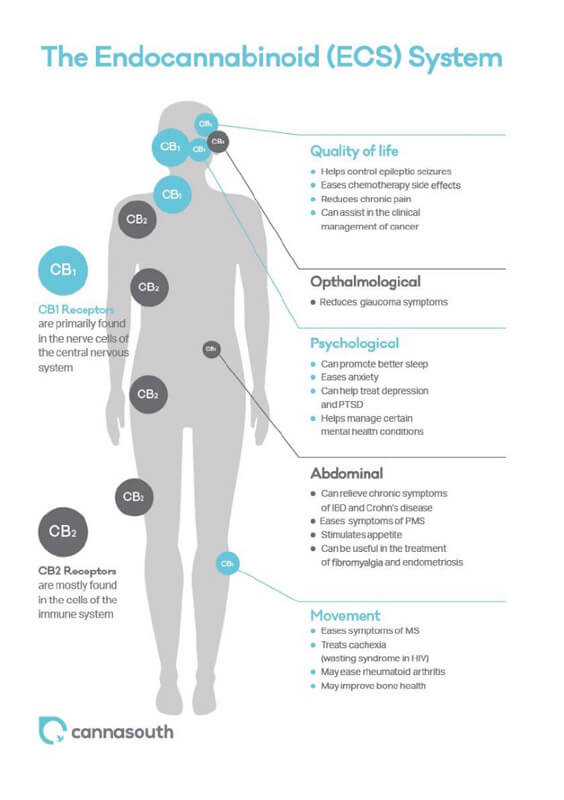 Since research tells us that deficiencies in the endocannabinoid system may be the cause of many medical conditions, it’s important that healthcare practitioners pharmacists have a good understanding of how best to support a healthy endocannabinoid system (ECS).
Since research tells us that deficiencies in the endocannabinoid system may be the cause of many medical conditions, it’s important that healthcare practitioners pharmacists have a good understanding of how best to support a healthy endocannabinoid system (ECS).
Like other transmission pathways, the endocannabinoid system (ECS) is comprised of receptors, ligands, and transport/degradation proteins. Endocannabinoids are the endogenous ligands that interact with cannabinoid receptors CB1 and CB2. The interaction between endocannabinoids and the CB1 and CB2 receptors help regulate important physiological processes involving the central nervous system, immune system, and metabolism. In fact, CB1 is among the most abundant receptor present in the central nervous system.
Our endocannabinoids e.g., anandamide (AEA) share structural similarities with cannabinoids found in the cannabis plant e.g., tetrahydrocannabinol (THC). By targeting the ECS system, medicinal cannabis extracts can help ameliorate ECS dysfunction and improve symptoms of multiple disorders such as obesity, vomiting, anorexia, multiple sclerosis, pain, inflammation, epilepsy, Parkinson’s disease, Huntington’s disease, Tourette’s syndrome, Alzheimer’s disease, Bipolar disorder, Schizophrenia, Post-Traumatic Stress Disorder (PTSD), depression, anxiety, insomnia, asthma, cardiovascular disorders, glaucoma, and cancer.
There are several online resources available on the ECS including a video Cannasouth has produced that walks you through this.
Where to find more information
Cannasouth has partnered with the Goodfellow Unit to develop a course on Medicinal Cannabis and Chronic Pain which covers the potential value of medicinal cannabis in chronic pain, how to start prescribing, who is likely to benefit, potential side effects and contraindications for prescribing. Upon completion of this short course, you can print your certificate for one (1) Continuing Professional Development (CPD) hour. There are also several more online and on-campus courses throughout New Zealand that can expand your knowledge further.
In addition to professional courses, we encourage you to explore our Clinical Fact Finder, an online suite of resources designed to support healthcare practitioners to quickly and easily find clinical evidence on the use of medicinal cannabis. All research has been fact-checked by Cannasouth’s team of scientists. The Clinical Fact Finder also contains reviews undertaken by Heath Agencies around the world on medicinal cannabis and its use in clinical practice. Here is a short video demonstrating how to use the Clinical Fact Finder.
Finally, at last year’s Medsafe event, Cannasouth received feedback from healthcare practitioners requesting a short guide that details common conditions and which products may be suitable treatment options. If you would like a copy of the guide, please email us at enquiries@cannasouth.co.nz and a member of our team will have this sent to you.
Cannasouth enjoys interacting with our social media audience and engaging in thoughtful discussion.
At Cannasouth we encourage an inclusive, informed and engaged online community. We feel fortunate to be involved in the medicinal cannabis industry in New Zealand and know that we have a passionate audience who come from a wide variety of backgrounds, with a vast range of different views and life experiences. We also understand that due to the history of prohibition surrounding medicinal cannabis, many patients and caregivers are frustrated with the history of or continued lack of access to medicinal cannabis products.
Cannasouth operates in a highly regulated sector and is a publicly listed company. We also produce products that are in most jurisdictions, including New Zealand, controlled drugs, or prescription medicines. As such we cannot discuss products specifically or certain company-specific information that may be deemed commercially sensitive. There may be questions that we will not answer in a public forum for these reasons.
To ensure we provide a safe place for both our employees and social media audience to engage we have community guidelines that we reserve the right to enforce.
Our social media moderators may delete or hide posts or block users that do not follow these guidelines:
- Be respectful. Please use common courtesy and do not make comments that contain offensive, profane, defamatory, racist, homophobic, threatening language or which are otherwise inappropriate in a public forum. These comments will be removed in accordance with the Harmful Digital Communications Act.
- History of abusive or threatening behaviour. If we believe a user or alias of a user has a history of online abuse or threatening behaviour, we may either hide or delete their comment and or block that user account to protect our employees and other users of our social media accounts.
- Do not spam us or troll us. Making the same point over and over either on the same or different posts is considered spamming or trolling. If this happens, we may leave your first comment but hide or delete the duplicates. Repeated instances will result in a user being blocked.
- Stay on topic. Only make comments that are relevant to the topic or theme of the post. If you disagree with our view on something and decide to make that point every time we post, your comments will be considered spam/trolling.
- Commercially motivated. If we believe a comment is commercially motived either to promote another company or product and or deliberately discredit Cannasouth or another company, we may delete or hide that comment. Repeated instances will result in a user being blocked.
- Creating a conversation. We often share links to third-party websites to encourage thoughts and discussions. This does not mean we endorse the views expressed on the third-party website. We also don’t necessarily endorse the views expressed by others on our social media channels.
- Give credit where credit is due. When you share an interesting image or video from our page/s, please take the time to tag us or include the handle responsible for the original post.
- Protect your own and others’ privacy. Never post comments containing personal, identifying, or confidential information such as account details or other personal information including address, telephone number, email, passwords, etc.
- Do not post advertisements or solicitations on our comments section. Do not use fake accounts or ‘bots’ to troll our pages. Do not tag us in profane and offensive posts or tweets. Such tags will be removed. Repeated instances will result in a user being blocked.
- We are a publicly listed company. This means we must comply with our Market Disclosure Policy which can be found here: https://www.cannasouth.co.nz/investors/governance/
Spreading information, which is not released to the market, whether true, false or otherwise can lead to serious charges and criminal convictions. Comments or posts which are made that put us in breach of this policy will be deleted immediately and may be reported to the relevant authorities.To allow our online moderators to have some rest, we may limit the hours that comments can be posted or made visible on our social media platforms.Thank you for adhering to these guidelines.
If you have any queries or comments or wish to discuss anything directly with us, please email enquries@cannasouth.co.nz or send us a direct message on our social media platform.
These guidelines may be updated from time to time.
Last updated 17/11/2021
Cannasouth has appointed Tony Clark as Operations Manager of its Cannasouth Cultivation Limited joint venture business.
Mr Clark brings to Cannasouth more than 20 years of engineering, manufacturing, and pharmaceutical industry experience, having worked with Pentair Valves & Controls Pacific Pty Ltd and Douglas Manufacturing Ltd. He is currently the General Manager of Douglas and will join Cannasouth on 20 September 2021.
Clark will manage the company’s state-of-the-art controlled environment agriculture (CEA) sealed greenhouse and headhouse facility in the Waikato, which was recently completed and is now in the final phase of equipment installation and validation.
Cannasouth CEO Mark Lucas says his team is excited to attract talent of Mr Clark’s caliber to the business.
“We are fortunate to have attracted some exceptionally skilled and knowledgeable people to our business. We have assembled a world class team. With our senior executive team now in place, Tony’s appointment represents one of the final pieces of the puzzle.
“Cannasouth Cultivation has an existing team of cultivation and production specialists who have been working closely with our Colorado based partner, Vera Cultivation, on the design and implementation of our commercial cultivation operations. Tony’s appointment as Operations Manager will be key to providing leadership and expertise across all aspects of our cultivation business.
“The design of our innovative facility represents the next generation of cannabis cultivation operations. It has been set up to produce premium pharmaceutical quality biomass at a highly competitive production cost, whilst being more energy efficient and environmentally sustainable than indoor cultivation operations.
“Tony understands the importance of a culture that prioritises the health and safety of its people. He will also provide leadership to environmental sustainability and responsibility, which are key values of Cannasouth.”
Mr Clark’s first priority will be preparing for a successful first commercial scale harvest scheduled for the end of this year. He will also be focused on leading the team towards achieving Good Agricultural and Collection Practices (GACP) and Good Manufacturing Practice (GMP) Certification. There is strong global demand for premium pharmaceutical quality cannabis flower, which will provide lucrative export opportunities for Cannasouth.
Mr Clark says Cannasouth is at the forefront of an emerging industry and he is excited to be joining the team.
“Cannasouth’s priority is to improve patient access to medicinal grade cannabis products that contribute to better health outcomes for New Zealanders. I am looking forward to being part of the team that will make that happen.”
Last week, Cannasouth released its plans to purchase the remaining 50% stake in Cannasouth Cultivation Limited it does not already own from Aaron Craig and his family interests.
Leading medicinal cannabis company, Cannasouth Limited has today entered into two conditional agreements to acquire the balance of the stakes that it does not already own in its cultivation and manufacturing joint venture businesses.
Acquisition of outstanding interest in Cannasouth Cultivation Limited
Cannasouth has entered into a conditional agreement with Aaron Craig and his family interests (Craig Family Interests) to acquire the remaining 50% stake in Joint Venture business Cannasouth Cultivation Limited that Cannasouth does not already own.
Cannasouth Cultivation has built a state-of-the-art growing and processing facility that will produce medicinal cannabis flower biomass at highly competitive production cost. It is energy efficient and more environmentally sustainable than indoor cultivation operations.
The facility, designed to produce premium Good Agricultural and Collection Practices (GACP) and Good Manufacturing Practice (GMP) pharmaceutical compliant biomass, is now in the equipment installation and validation phase. It is anticipated that the new facility will have sufficient capacity to generate circa $8 million of revenues per annum (based on conservative current global pricing), subject to Cannasouth Cultivation securing commercial offtake agreements and completing quality certification. The facility has been designed to be scalable.
Cannasouth Chief Executive Mark Lucas says, “There is strong global demand for premium pharmaceutical quality cannabis flower. The timing of the acquisition will bring all future revenues generated from the cultivation facility into the Cannasouth Group’s P&L. It also enables the Company to control all aspects of the cultivation operation.
“Cannasouth would like to take this opportunity to thank the Craig Family interests for their support and expertise as a valued joint venture partner in developing the innovative cultivation facility to such a high standard and in a cost-effective manner.”
The acquisition of the outstanding interest in Cannasouth Cultivation is conditional on the Company raising new capital by 30 September 2021through a capital raising initiative. Settlement for the acquisition of Cannasouth Cultivation is expected to happen shortly after this capital raise condition has been met.
Acquisition of outstanding interest in Midwest Pharmaceutics NZ Limited
Cannasouth has also entered into a conditional agreement with Mark Balchin and Greenmeadows Health Limited to acquire the remaining 40 per cent shareholding in Hawkes Bay-based Midwest Pharmaceutics NZ Limited that it does not already own.
Currently Cannasouth owns 60% of Midwest. The business generates circa $2 million per annum in revenues, which after the completion of the transaction will be consolidated into Cannasouth’s P&L from the date of acquisition.
Mark Lucas says, “The Midwest acquisition provides Cannasouth with the opportunity to streamline operations and generate additional revenues from existing operations, while positioning the business for GMP certified medicinal cannabis manufacturing.”
CEO of Midwest, Mark Balchin, will continue as Chief Manufacturing Officer for the Group.
The Midwest transaction is conditional on the parties obtaining the approvals of several landlords and regulators to the change in control of Midwest on or before 31 August 2021.
Commercial Benefits of the Acquisitions
- Take 100 per cent control of both joint venture cultivation and manufacturing businesses.
- Accelerate pathway to significant revenue generation.
- Improve operational efficiencies across the Group.
- Post-acquisition revenues and 100 per cent of future profits will be consolidated into the Cannasouth Group P&L.
- Opportunity to generate additional revenues from existing operations at Midwest while positioning the business for GMP certified medicinal cannabis manufacturing.
Cannasouth is proposing a capital raising to fund the acquisitions. The Company is currently finalising its capital raising strategy and will release details to the market in due course.
Cannasouth Limited has released a Medicinal Cannabis Industry Update.
The update is designed to share information about the emerging medicinal cannabis industry both in New Zealand and globally; the opportunities and challenges facing industry players, the New Zealand regulatory environment, and the barriers to entry.
CEO Mark Lucas says: “There is a lot of confusion and a lack of understanding about what is a highly complex and regulated industry. Releasing this update is an opportunity for Cannasouth to share its knowledge and expertise and provide our investors, stakeholders and the wider public with some clarity around the myths and misconceptions of the industry.”
The update is presented in 8 sections:
1. Introduction
2. Products and Services
3. Product Quality: Pharma Vs non-Pharma
4. Patients: Challenges and Opportunities
5. Geographic Markets
6. Cultivation and Pharmaceutical Manufacturing
7. Regulatory Environment in New Zealand
8. Closing Remarks
Cannasouth is preparing a follow-up to this update. This information will focus on how it is working to create genuine advances in the medicinal cannabis industry, insight into Cannasouth’s growth strategy, timelines for implementation, and exploring the competitive advantage that makes it a leader in the New Zealand market.
The comprehensive document is available to view and download here:
Cannasouth has recently been approved for a new Fellowship Grant from Callaghan Innovation to support a Masters research study in conjunction with the University of Waikato to create a library of cannabis-derived biomolecules.
The cannabis plant contains several hundred known molecules and likely many more unique and as yet unidentified compounds of interest. This library of biomolecules can be used in computational molecular docking studies allowing Cannasouth to investigate potential pharmacological activity of selected molecules prior to undertaking complex chemical extraction and isolation processes of these minor compounds. This approach aids fast-track assessment of new compounds in terms of safety and efficacy leading to the potential for new medicines and Intellectual Property.
Chief Science Officer David Gill says, “it is important to build strong, focussed, scientific collaborations with our leading academic institutes and these grants enable Cannasouth to undertake cutting edge research into next generation cannabis related therapeutics in parallel with our goal of delivering products to the patients who need them now.”
Cannasouth is also conducting a neuropathic pain / drug delivery PhD project with support from Callaghan Innovation. In addition, Cannasouth recently completed a Masters research study focusing on Tissue Culture research providing Cannasouth with new knowledge related to micropropagation techniques to support commercial growing activities. The Masters student has since been employed in a new R&D role supported by a Callaghan Innovation Careers Grant.
CEO Mark Lucas says, “Cannasouth actively works with government agencies such as Callaghan Innovation to support research and development opportunities both in-house and with our research partners. We are pleased to be part of developing future generation scientists of New Zealand and offer them a career pathway in the emerging medicinal cannabis industry.
“It is rewarding to know that Callaghan is funding these research activities to create opportunities to discover unique IP and help advance this emerging industry sector in New Zealand.”
Please click on the link below for our formal Bank change confirmation.
Bank change confirmation letter
The range of conditions that can be managed with medical cannabis is increasing exponentially, this article aims to give a brief overview of what the basics of medical cannabis are, which illnesses it may help, and how it is prescribed.
Are you a doctor considering prescribing cannabis? or a patient suffering from a medical issue that cannabis can help manage? Would you like more information on medical cannabis? If you answered “yes” to any of these questions, read on.
What is medical cannabis?
In very simple terms, medical cannabis is prescribed specifically to alleviate the symptoms of an illness. In New Zealand, medical cannabis needs to be prescribed by a doctor for a particular condition.
The therapeutic properties of the cannabis plant come mainly from the cannabinoids naturally occurring in the plant. There are between 80 and 144 cannabinoids present in the cannabis plant- the two main cannabinoids that have been found to have therapeutic effects are tetrahydrocannabinol (THC) and cannabidiol (CBD). Many of the therapeutic effects of THC and CBD are thought to be the result of their interactions with the endocannabinoid system.
The endocannabinoid system (ECS) is a complex cell-signaling system discovered in the 1990s. As a result of this discovery, it was found that the ECS regulates multiple physiological processes involving the immune system, the nervous system, and metabolism. It also discovered cannabinoid receptors (CB1 and CB2) are scattered throughout your central and peripheral nervous system. Additionally, it found that naturally occurring cannabinoids (endocannabinoids) bind to the CB1 and CB2 receptors to exert an influence on various physiological processes. Furthermore, it was established that exogenous cannabinoids such as THC and CBD were able to bind to cannabinoid receptors in a similar way to endogenous cannabinoids.
THC is responsible for the ‘high’ often associated with cannabis due to its psychoactive properties. CBD does not create a ‘high’ and, when taken in conjunction with THC, can mitigate some of THC’s negative effects such as anxiety.
Medical cannabis can be specifically formulated to contain different ratios of THC:CBD allowing prescribers to tailor each patient’s treatment depending on their condition. This is in contrast to recreational cannabis, which is unregulated, and often contains very high levels of THC in order to maximise the ‘high’.
What conditions can medical cannabis treat?
The list of conditions that have shown benefits from medical cannabis is growing rapidly as more and more research is being published. Therefore, the following list will grow considerably as the plant is more widely studied and prescribed.
There is currently evidence for the use of medical cannabis for the following conditions:
Condition |
Evidence |
Epilepsy |
There is ample clinical evidence for the use of medical cannabis in the management of refractory epilepsy, especially in childhood. |
Multiple sclerosis (MS) |
There is clinical evidence for the use of medical cannabis in the management of the pain and spasticity of MS as well as the urinary dysfunction associated with MS. |
Osteoarthritis |
There is pre-clinical and clinical evidence to show that medical cannabis alleviates the pain associated with osteoarthritis. |
Chronic pain |
There is clinical evidence supporting the use of medical cannabis in the management of chronic pain (cancer and non-cancer chronic pain) as well as painful spasticity. Medical cannabis may also have opioid-sparing properties in the management of chronic pain. |
Psychiatric disorders |
There is clinical evidence to support the use of medical cannabis in the following psychiatric disorders: anxiety, social anxiety, insomnia, post-traumatic stress disorder, and schizophrenia (as adjunctive therapy). |
Chemotherapy-induced refractory nausea and vomiting |
There is clinical evidence that shows medical cannabis can decrease the symptoms of nausea and vomiting associated with chemotherapy. |
Insomnia |
Medical cannabis has been shown to assist with improving sleep quality, decreasing sleep disturbances, and decreasing sleep onset latency. |
Weight gain in patients with cancer, HIV, or undergoing palliative care |
Some benefit has been shown in using medical cannabis to assist with increasing appetite in patients with cancer or HIV. |
Cancer |
In vitro and preclinical trials have shown inhibition of the growth of certain tumour cells in response to cannabinoids. |
Rheumatoid arthritis (RA) |
Preclinical trials have indicated a place for cannabinoids in the management of RA as a result of their anti-inflammatory properties. |
Chronic pruritus (associated with contact dermatitis, atopic dermatitis, eczema, psoriasis) |
Multiple pre-clinical and some preliminary clinical trials have shown medical cannabis to be effective in reducing intractable pruritus. |
The range of medical conditions that respond to medicinal cannabis is likely to increase as further research takes place.
Prescribing medical cannabis
Currently, medicinal cannabis is available in New Zealand only on a doctor’s prescription. According to the BPACNZ better medicine website, as of 1 April 2020, any practitioner can now prescribe any medicinal cannabis product. These products must meet the minimum quality standards set by the Medicinal Cannabis Agency and can be prescribed for any indication within their scope of practice and where there is a clinical need.
It is important to remember the maxim ‘start low, go slow’ when initiating treatment with medical cannabis.
What to look for when prescribing medical cannabis products
The specifications surrounding the production and manufacture of medical cannabis in New Zealand stipulate that the products must comply with Good Manufacturing Practices (GMP). Therefore, these products are amongst the highest quality medicinal cannabis products available globally. This means that all registered products are extremely well-regulated. Therefore, registration of a product by Medsafe ensures that there is adequate safety and efficacy data available for the product.
There is more than just safety and efficacy to consider when prescribing medical cannabis, however.
The following also need to be considered:
- Start low and go slow: It is important to remember the maxim ‘start low, go slow’ when initiating treatment with medical cannabis. Tolerance to THC is patient-dependent depending on a range of factors including age, metabolism, pre-existing illnesses/medications, and prior THC use. Therefore, starting at a low dose and titrating up as necessary to achieve the desired result ensures that any potential side effects are avoided or minimised.
- Delivery method: Medical cannabis products come in various delivery methods, such as oils, sublingual sprays, capsules, creams, etc. Therefore consider what is available, which delivery methods are acceptable and which delivery methods are most efficacious for the medical condition being treated.
- Formulation: Medical cannabis can potentially come in different ratios of THC:CBD. Ideally, one would want to be able to choose from the following: a high THC: low CBD ratio, a balanced THC:CBD ratio, and a high CBD: low THC ratio, depending on what medical condition is being treated. It is also useful to have CBD-only formulations for those patients who are wary of THC or when THC is contraindicated. The following table can give an indication of various uses of different ratios of THC:CBD:
- Cost: Certain medical cannabis products on the market are prohibitively expensive, so check the cost before making a decision on a product.
Condition |
Ratio of THC:CBD |
Moderate pain, e.g. arthritis type pain
|
Choosing a high CBD:low THC ratio allows for pain relief without impacting functioning or mental acuity. |
Severe pain, e.g. neuropathic pain and cancer pain
|
Severe pain responds better to either balanced THC:CBD formulations or formulations with a higher THC:lower CBD ratio. When using a higher THC component, remember the ‘start low, go slow’ principle as patients will all have a different tolerance to THC depending on age, metabolism, prior THC use etc. |
Anxiety
|
Anxiety responds better to a balanced THC:CBD ratio or a higher CBD:lower THC ratio. In addition, CBD alone is also effective for the management of anxiety. |
Chronic insomnia
|
THC has well-recognized sedating effects but can possibly increase anxiety. Adding CBD can mitigate increased anxiety. Therefore you could choose either a balanced THC:CBD formulation or a higher CBD: lower THC formulation. |
Chemotherapy induced nausea and vomiting
|
Choose a balanced THC:CBD or higher THC:lower CBD formulation. |
Spasticity (especially related to MS)
|
Studies were done using a balanced THC:CBD formulation (1:1 ratio). |
How safe is medical cannabis?
It is reassuring to know that medical cannabis is well-tolerated and generally considered safe. Furthermore, a 2020 review of the scientific evidence for the use of THC:CBD oromucosal spray for the management of chronic pain showed no new safety concerns with long-term THC:CBD use. The review also showed no evidence of dependence. This finding supported the findings of a 2019 study that found medical cannabis, especially CBD-only formulations, to have minimal side effects.
Although medical cannabis is a very safe option for most people, there are some contra-indications to be aware of. THC and CBD are metabolised via the cytochrome P450 (CYP450) pathway. As a result, medical cannabis is contraindicated in people with disorders of CYP450 enzymes.
Medical cannabis can interact with certain medications so care should be taken when medical cannabis is prescribed with any of the following medications: warfarin, ketoconazole, clobazam, theophylline, olanzapine, and clozapine, opioids, anticholinergics, and certain antihistamines.
In summary
Patients can use medicinal cannabis for the treatment of a growing list of medical conditions. Due to that growth, this list will likely expand as more research is published. Furthermore, medical cannabis is considered safe and is generally well-tolerated with little risk of dependence.
The strict regulations in place around the registration of medical cannabis products in New Zealand should ensure that doctors feel confident when prescribing medical cannabis that they are prescribing a safe and efficacious product. You should take care when prescribing to choose the optimum delivery method and formulation specific to your patient.
Pain is the number one indication treated by medical cannabis, with over 70% of prescriptions made for pain-related symptoms.
Now that patients can legally access medical cannabis in New Zealand, it’s more important than ever for doctors to understand how cannabis works for pain.
Here’s everything you need to know.
What is medical cannabis?
The term “medical cannabis” refers to cannabis products containing the cannabinoids tetrahydrocannabinol (THC), cannabidiol (CBD), or a combination of both, specifically used for the management of medical conditions. Medical cannabis can also refer to synthetic cannabinoids and “minor cannabinoids” such as cannabigerol (CBG) and cannabinol (CBN).
Cannabis sativa contains around 113 chemical compounds known as cannabinoids. The most commonly known and studied of these are THC and CBD. THC is responsible for, among other effects, the intoxicating and psychoactive effects of cannabis. CBD does not have the same intoxicating “high” like THC. When taken together at suitable ratios, CBD may mitigate some of the intoxicating effects of THC.
Although this article primarily looks at medical cannabis to alleviate pain, it has been widely studied and has shown to be effective in helping with a wide range of other medical conditions as well.
How medical cannabis works to alleviate pain
Extensive preclinical and a few clinical studies suggest promising pain-relieving properties of THC and CBD. Until recently, studies assessing the use of medical cannabis were limited due to legal and accessibility issues surrounding medical cannabis. As the use of medicinal cannabis is legalised and becomes more widespread, more extensive clinical trials will likely be performed. These will help to determine the efficacy and safety of medical cannabis.
Animal models show medical cannabis has significant analgesic (pain-relieving) effects on inflammatory, neuropathic and arthritis-related pain. Many of these studies have investigated the mechanism of action of THC and CBD in pain relief.
Researchers believe it is primarily through the interaction with the endocannabinoid system (ECS) that medical cannabis works to reduce pain.
The ECS is a complex biological system comprised of cannabinoid receptors (CB1 and CB2) that regulates many physiological processes, including immunity, appetite, metabolism, and more.
How THC can alleviate pain
THC acts in a similar way to endogenous endocannabinoids to activate the CB1 and CB2 receptors, which is how it’s thought to affect pain.
Activation of the endocannabinoid system by THC causes the inhibition of ascending nociceptive transmission (pain perception). It also causes the activation of the inhibitory descending pain pathways and the modulation of the emotional component of pain. This happens through the activation of CB1 receptors in the limbic and cortical areas of the brain. The overall effect is to decrease the patient’s sensation of pain.
In chronic pain conditions, there is often increased sensitisation to pain. The increased sensitisation means that the more pain individuals experience, the more sensitised they become to feeling pain. This becomes a pain cycle that is hard to break.
Activation of peripheral and central CB2 receptors modulate immune and inflammatory responses, leading to neuronal sensitisation inhibition in chronic pain. THC can intercept the cycle of increased pain sensitisation.
How CBD can alleviate pain
The interaction of CBD on the endocannabinoid system is not as straightforward.
CBD is an allosteric inhibitor of THC binding to CB1. That means CBD modifies how THC binds to CB1 receptors to lessen THC’s stimulatory effect on the receptors. Through this mechanism of action, CBD, when combined with THC in medical cannabis, alleviates the psychoactive effects of THC; lessening the “high” patients might feel.
CBD is an inverse agonist of CB1 and CB2. This means that when CBD binds to CB1 and CB2 receptors, it inhibits rather than activates them.
Typically, activation of CB2 leads to cytokine release, which causes the migration of immune cells to target sites. This is part of the normal inflammatory pathway and is necessary for normal immune function, wound healing, and more.
However, it becomes problematic when the inflammatory response is exaggerated, as it is in certain pain conditions such as arthritis. CBD binding to the CB2 receptor inhibits the inflammatory response, decreasing inflammation.
There are secondary pathways by which CBD can decrease pain. Some of the analgesic effects of CBD are mediated by the activation of the serotonergic 5HT1a receptors, key mediators in anxiety and depression. This pathway is important when considering the link between pain and depression.
CBD has also been shown to have sleep-promoting effects, which also plays a role in pain reduction and increases the quality of life of people suffering from painful conditions.
Conditions medical cannabis may treat
Multiple Sclerosis
Multiple Sclerosis (MS) is a degenerative disease that affects the central nervous system, especially the brain, the spinal cord, and the optic nerves. Symptoms can range from mild visual loss and weakness to severe, debilitating muscle spasms, loss of mobility, and paralysis.
Many clinical trials support medicinal cannabis in the treatment of the pain and spasticity associated with MS. A 2020 review of over 33 studies and 7500 patients found adequate data to support the safe and effective use of medical cannabis at a THC to CBD ratio of 1:1 for alleviating pain and spasticity in MS patients.
Neuropathic pain
Neuropathic pain is pain resulting from nerve damage. Often described as a severe burning sensation, it can result from trauma, diseases (e.g. diabetes, thyroid disease), or medication. It is also largely intractable pain and is notoriously unresponsive to standard pain medication.
A 2020 review of the use of medicinal cannabis in the management of pain found the most evidence to support its use in chronic neuropathic pain. Medical cannabis could be a welcome option for many who are unable to treat chronic neuropathic pain.
Arthritis-related pain
The pain from arthritis is a combination of inflammatory, nociceptive, and neuropathic pain. The articular cartilage of joints breaks down in patients with rheumatoid arthritis and osteoarthritis (primarily mediated by proinflammatory cytokines). Animal models have shown cannabinoids are effective in reducing joint damage from the breakdown of cartilage.
A 2019 review found a potential for the use of cannabinoids in managing rheumatoid arthritis by using a targeted receptor approach. Further clinical trials are required in this area to understand its full potential.
Cancer pain
A 2020 review of cannabinoid use in cancer patients showed promise for medical cannabis in the management of cancer pain. The results showed improved pain scores in patients with advanced-stage cancer when medical cannabis with a THC:CBD ratio of 1:1 was added to opioid treatment. An observational study also showed an improvement in pain and the quality of life. However, it was also mentioned that there is a need for further high-quality randomised controlled trials to adequately define the role of medical cannabis in managing pain in cancer patients.
Medical cannabis has not only been used directly for cancer pain but has also been used successfully in alleviating the side effects of chemotherapy. There is good evidence to show that medical cannabis can reduce nausea and vomiting associated with certain chemotherapy treatments. It can also help counteract the loss of appetite and wasting (cachexia) that many cancer patients suffer from.
Importantly, across all reviewed studies in patients with cancer-related and non-malignant pain who were using daily THC:CBD for an extended period, there were no new safety concerns identified. There was also no evidence of the development of tolerance to the medication i.e. the dose did not have to be increased with extended periods of use in order to have the same effect.
Opioid-sparing effect of medical cannabis
Opioids are usually the drug of choice for chronic, severe pain when first-line pain options (paracetamol and non-steroidal anti-inflammatories) have proved ineffective. Although opioids are effective, they come with a host of risks and side-effects. Most concerning, is their addictive potential, with up to 1 in 4 patients in primary care who have been prescribed opiates developing dependence.
Even without addiction, opiates have many unwanted and intolerable side-effects such as constipation, nausea, drowsiness, itching, depression and tolerance (meaning that with time, you need a continually higher dose to achieve the same level of pain control).
Medical cannabis has been shown to be opioid-sparing. This means that if a patient is already on opioid medication, by adding medical cannabis the patient is able to decrease their dose of opioids. This decreases both side-effects and the risk of dependence.
The bottom line
There is a growing body of evidence to support the use of medical cannabis in the management of pain. This is only likely to increase going forward as further legal restrictions on the use of medicinal cannabis are lifted.
Traditional methods of pain management come with many unwanted risks and side-effects and can often be lacking in efficacy. Medical cannabis could well fill the gap in pain management left by existing pain medications.
Even though New Zealanders can be prescribed legal products containing CBD (cannabidiol) and THC (tetrahydrocannabinol), there is still widespread use of illegal-market cannabis to treat medical symptoms.
This article will discuss some of the inherent risks of using illegal-market cannabis for medicinal purposes and the benefits of regulated medicinal cannabis.
Reasons for illegal market medicinal cannabis use
While doctors can prescribe medicinal cannabis products, there are various reasons why patients may turn to illegal cannabis-based alternatives for medicinal purposes.
Firstly, most doctors are not comfortable prescribing medicinal cannabis. This is largely due to inadequate knowledge and/or training in the effects of medicinal cannabis. It has been suggested that only about a quarter of general practitioners in New Zealand felt that they knew enough about medicinal cannabis to feel comfortable prescribing it. Similarly, many patients are not aware of the availability of legal medicinal cannabis or its potential applications.
Cost is also a significant hurdle that restricts patient access to medicinal cannabis products. Legal production of medicinal cannabis must be completed under strict guidelines such as good manufacturing practice (GMP). They must also adhere to the strict medicinal cannabis scheme’s quality guidelines. This results in high costs of manufacture and hence expensive products for patients. Furthermore, it is unlikely that Pharmac will subsidise medicinal cannabis products; hence, legal medicinal cannabis may remain significantly more expensive than its illegal-market counterparts.
Risks of illegal market medicinal cannabis use
The use of illegal-market cannabis is, by its very nature, unregulated both on the manufacture and consumer/user level. There is no patient selection, meaning people with contraindications to cannabis may not be appropriately excluded. Paying for a script to get a regulated cannabis product may cost more than following the self-prescription route, but it ensures there are no contraindications to cannabis use and also that there is a valid indication cannabis can treat.
There are also no product regulations in illegal-market medicinal cannabis, so doses may not be accurate or reproducible. The temptation to buy homegrown cannabis products is very real because they are usually cheaper. However, the lack of regulation of either active ingredients (relative levels of CBD and THC or terpenes) or contaminants such as mycotoxins or heavy metals may result in harm to the end-user.
There is the risk when using illegal-market cannabis products that the therapeutic end goal is not met, not because medicinal cannabis is ineffective, but because the active ingredients in unregulated products have varied concentrations and in some cases may not be present at all.
Benefits of regulated medical cannabis
The Medicinal Cannabis Agency oversees the Medicinal Cannabis Scheme and ensures that medicinal cannabis products meet a minimum standard of quality. The Medicinal Cannabis Scheme enables the commercial cultivation of cannabis for medicinal use and regulates the manufacture and supply of cannabis-based products.
A series of upper limits, restrictions, forms, and processes that must be adhered to are also defined by this scheme. In practical terms, this means final medicinal cannabis products must meet the Minimum Quality Standards set by the Ministry of Health.
Contamination
The minimum quality standard sets testing requirements and maximum limits (e.g., limits for microbial contamination) that medicinal cannabis products and ingredients must comply with.
Medicinal cannabis must be tested for:
- Active compound concentrations
- Microbial contamination
- Heavy metals
- Pesticides
- Absence of aflatoxins
- Ochratoxin A
- Foreign matter
- Loss on drying
- Total ash
- Residual solvents
This regulation ensures that the final product is not contaminated with elements that could cause adverse reactions. There is a risk with homegrown products that there could be microscopic, unrecognised contamination e.g. aflatoxins, and these could result in adverse reactions ranging from rashes to anaphylaxis. Regulating contaminants negates this risk.
Other quality requirements
The Minimum Quality Standard sets a standardised list of requirements that are essential for regulating the quality of a medicinal cannabis product. These ensure the product contains the correct ingredients, is consistent through its shelf life, is packaged and labelled appropriately and does not contain any ingredients that could pose a risk of harm.
Active ingredients
For the purposes of the minimum quality standard, the active ingredients in cannabis-based ingredients and medicinal cannabis products are:
- Delta-9-tetrahydrocannabinol (THC) and its corresponding acid, delta-9-tetrahydrocannabinolic acid (THCA)
- Cannabidiol (CBD) and its corresponding acid, cannabidiolic acid (CBDA)
- Any other ingredient derived from cannabis and whose stated content is at least 1.0% of the ingredient or product by weight or volume
This regulation ensures that the amount of active ingredient in the product is consistent with an expected therapeutic endpoint. There is a risk with homemade products that they could theoretically contain very little, no, or in some cases, dangerously high levels of an active ingredient.
Shelf life and storage conditions
For the entirety of its shelf life, the ingredient or product must remain compliant with the minimum quality standard requirements for:
- Microbiological contamination
- Loss on drying
- Assay limits for active ingredients
- Form and dosage form
Although homemade products may be adequate immediately after production, there is a risk the product may degrade or become compromised due to incorrect handling, packaging, or storage.
With exposure to the elements, there may be degradation of active ingredients, making the medication less effective, and increasing the risk of contamination. Regulated products, on the other hand, have specific requirements for packaging and storage that need to be met in order to ensure that the product does not degrade or become contaminated. There is control over product batches as well as expiration dates.
Container material
The container for a cannabis-based ingredient or medicinal cannabis product must be regulated, safe, and meet minimum quality requirements. An inferiorly made container can lead to contamination of the cannabis product as well as product degradation. Ensuring that the container meets certain requirements is also important to prevent accidental overdose and tampering by children i.e., child-resistant packaging.
Labelling
Medicinal cannabis products must meet the packaging and labelling requirements for medicines. This is very important, and one of the very obvious factors that delineate regulated cannabis products from unregulated ones.
Correct labelling allows for more accurate prescribing and dispensing. The label should state levels of active and inactive ingredients, ensuring that the prescribing doctor, the dispensing pharmacist and the patient all know precisely what has been prescribed and is being taken. It is unlikely that homemade products would achieve this.
Dosage form
Medicinal cannabis products (including CBD products) must be in a pharmaceutical dosage form that is regulated and standardised. The importance of this cannot be overstated. The risk with illegal-market products is the potential to receive inconsistent dosing even with the same material. This means that in addition to each product having potentially significantly different ingredients, the amount consumed in each dose may also be inconsistent.
When the product’s manufacturing is regulated so that dosage forms are standardised, doctors and patients can be assured of more predictable responses. This is imperative for achieving therapeutic control.
Restrictions to control contamination, adulteration and form
The minimum quality standard includes restrictions to control any contamination or adulteration and the form of medicinal cannabis products. This may be as simple as a seal on a product, to ensure that the product has not been tampered with or adulterated. Unfortunately, because of the cost involved in these extra safety steps, homemade products do not necessarily include them; however, they are important for patient safety.
Good Manufacturing Practice
Good Manufacturing Practice (GMP) is the term used to describe the systems that manufacturers of medications have in place to ensure that their products are consistently safe, effective and of acceptable quality. Medsafe uses GMP regulations to determine approval of medications. GMP is not specific to medicinal cannabis products, but by choosing cannabis products that follow GMP, doctors can be confident in the quality and safety of the product.
GMP ensures, among other things:
- The product is of a certain quality and that it contains the active ingredients that it claims to
- That the levels of active ingredient are consistent; that the product is safe and is not contaminated
- and that the sourcing, manufacture and distribution of the product adhere to standardised protocols.
A product that is regulated under the principles of GMP drastically reduces the inherent risks of illegal-market or unregulated products.
Why is medical cannabis better off being legal?
There is ample evidence to support the safety and efficacy of medical cannabis. A legal medicinal cannabis market allows for careful patient selection. This excludes patients with risk factors for, or contraindications to, medicinal cannabis. It also provides a framework for consistent medicines. Choosing prescribed medicinal cannabis mitigates risks associated with an illegal unregulated medicinal cannabis market.
Prescribed medicinal cannabis products are backed up by the quality process and strict regulations required by GMP. These regulations and processes ensures that the product contains the active ingredients that it claims to. The product has been through rigorous testing and has the concentration and ratio stated on its label.
Illegal-market medicinal cannabis may well be cheaper in the short term because of the costs associated with GMP compliance. Patients benefit from the safety and predictable therapeutic outcomes in the long-term, however, when using regulated medicinal cannabis products these benefits far outweigh the cost difference.
The use of cannabis for medicinal purposes is becoming more and more accepted throughout many countries. With the increased use of these medicines, the CBD:THC ratio is becoming more important. As popularity grows, research is being done to further understand the therapeutic benefits of different combination formulations and doses.
Currently, in New Zealand, only one medicinal cannabis product (Sativex) has been approved by Medsafe. This is for use as an add-on treatment for the symptoms of moderate to severe spasticity due to multiple sclerosis (MS). Sativex contains equal amounts of CBD and THC (a 1:1 ratio).
However, a broader range of CBD:THC combinations are being investigated to treat other indications, such as pain, epilepsy, anxiety, sleep disorders, etc. These combinations range from CBD-dominant to THC-dominant, for example, CBD:THC 20:1 through to CBD:THC 1:10.
Understanding which combination to use on individual patients is challenging. The following sections provide guidance to help understand how the ratio of CBD:THC works so that going forward you can improve the effectiveness of medicinal cannabis.
Understanding CBD and THC
Cannabidiol (CBD) and tetrahydrocannabinol (THC) are both natural compounds found in plants of the Cannabis genus. Although both compounds interact with the body’s endocannabinoid system, the effects of these two compounds are very different. THC binds to CB1 receptors in the brain and causes feelings of euphoria or ‘a high’. CBD binds weakly to the CB1 receptor and only when THC is present. CBD does not produce euphoria or ‘a high’.
CBD and THC can be combined in medicinal products in different ratios to produce different therapeutic effects. Because the use of medical cannabis is a relatively new area in medicine, there is still a lot of research to be done in this area. We are still learning what ratios are best for managing various conditions.
What is a CBD:THC ratio?
The ratio of CBD to THC indicates the amount of CBD compared to the amount of THC in a dose. For example, a ratio of 1:1 would mean that the amount of CBD and THC are the same in each dose. A CBD:THC ratio of 20:1 would mean that there is 20 times the amount of CBD in a dose compared to THC. Changing the ratio of CBD to THC allows for a tailor-made product that utilises the unique effects of either CBD, THC, or both for a particular patient or clinical effect.
What are the most common ratios?
Cannabis cultivars grown for the recreational market have seen a steady increase in THC content, paired with a decrease in CBD. Smoking these varieties will give the user a more potent ‘high’, but this might come at the cost of some therapeutic effects.
Medical use of CBD and THC has changed that, allowing specific ratios of CBD:THC to be selected to provide the greatest clinical benefit for each patient. Now, most medicinal cannabis products are higher in CBD than THC. Pure CBD products are considered to have less than 0.3% THC. The trend has moved towards cultivating plants or producing products that have a higher CBD to THC ratio. Ratios of CBD:THC can range between >20:1 all the way to 1:10. As a general rule of thumb, anything higher than a CBD:THC ratio of 10:1 should not elicit a high.
CBD:THC ratio for pain
By changing the ratio of CBD to THC, you are able to target and manage different types of pain.
Mild to moderate pain due to inflammation (think arthritis-type pain) can be managed well with CBD-dominant products such as CBD:THC 20:1 and 10:1. These ratios will be unlikely to induce any intoxicating effects.
Neuropathic pain, from disease or damage to the nervous system, might be better treated by increasing the ratio of THC towards a balanced ratio, 1:1. The exact ratio will depend on the severity of the condition and how well THC can be tolerated by the patient.
Very severe pain, such as cancer pain, may require THC-dominant medications. It is important to understand that THC-dominant products may induce euphoria and sedation, so care needs to be taken.
CBD:THC ratio for anxiety
Smoking cannabis can induce paranoia or extreme anxiety in certain people, so it seems counterintuitive that you could use medical cannabis preparations for the management of anxiety.
CBD has demonstrated efficacy in treating various forms of anxiety and is commonly used for this purpose. However, recreational use of high-THC cannabis is associated with increased anxiety, particularly in high doses. Reports suggest that THC has a dose-dependent effect on anxiety, where at low doses THC may be anxiolytic (reduce anxiety) but at higher doses can be anxiogenic (cause anxiety). Therefore, CBD-dominant products (CBD:THC >10:1) are likely to provide the most beneficial treatment, and in some cases, pure CBD with no THC present may be the best product to treat anxiety.
CBD:THC ratio for insomnia
Insomnia is a widespread problem and has been linked to illnesses ranging from depression to cardiovascular disease to dementia. Many allopathic medications used in the management of insomnia have adverse side effects, such as daytime drowsiness or addiction.
THC is well-known to have sedating properties via its action at the CB1 receptor; however, its use alone can cause increased anxiety and lead to other undesirable effects. By adjusting the ratio of CBD:THC, it is possible to block these undesirable effects while still retaining sedating properties. A current study is looking at a CBD:THC ratio of 20:1 in the treatment of chronic insomnia. If you are taking medicinal cannabis for other indications, it may be possible to investigate adding a slightly higher dose of THC at night-time to maximise the sedating effects and reverting to the lower THC ratio for daytime use.
How to pick the best CBD:THC ratio for your patient

This infographic provides ratios of CBD:THC and considerations of which ratio to use for certain conditions.
At the time this blog was published (January 2021), the only available medical cannabis in New Zealand contains a ratio of CBD:THC at 1:1; however, going forward you will be able to prescribe your patient other preparations of CBD:THC at a ratio that is optimal for what they want to achieve. The following is a rough guide of what to expect from different ratios:
CBD:THC at a ratio of 1:2
This preparation contains twice as much THC as CBD and will have intoxicating effects, especially for new or naive users. The presence of some CBD in the preparation will dampen some side effects of the higher THC, such as paranoia, but not all. This ratio would be better suited for people who have been using medical cannabis on a chronic basis, e.g., for intractable nausea, poor appetite, or glaucoma, and have developed a high degree of tolerance.
CBD:THC at a ratio of 1:1
This preparation contains equal amounts of CBD and THC and, depending on the dose, is likely to cause symptoms of euphoria or intoxication, especially in people who are naive to cannabis use. If using this ratio, it would be prudent to start with low doses and increase slowly according to tolerance.
CBD:THC at a ratio of between 2:1 and 4:1
Preparations with this ratio of CBD to THC can be psychoactive, especially to people who have poor tolerance for THC. The increased CBD does have beneficial effects and causes some dampening of the effects of THC.
CBD:THC at a ratio of more than 10:1
CBD:THC ratios >10:1 generally produce no intoxicating effects and are ideal for patients that are not able to take THC during the day (e.g., due to driving or work). Where the condition to be treated does not require THC, these products may help provide relief for certain conditions without any intoxication of the patient. This is a really safe dose for those people who want to experience the beneficial effects of CBD without the psychoactive effects of THC.
The bottom line
There is definitely an advantage to being able to adjust the ratio of CBD:THC in your medical cannabis preparation. This will allow you to maximise the particular benefits that you want while minimising any unwanted negative side effects. Currently, the standard preparation contains a fixed ratio of CBD:THC; however, going forward new research may strengthen the argument for flexible dosing.
THC is psychoactive and may impair your ability to undertake certain tasks, such as driving or operating machinery. CBD is non-psychoactive and non-sedating and can be safely used at much higher doses than THC.
With all medicinal cannabis, it is important to start with a low dose and gradually increase it over a few days. This is particularly important for compositions containing THC. Remember: START LOW and GO SLOW.
Cannasouth Limited’s joint venture partner Cannasouth Cultivation Limited has been granted its commercial cannabis cultivation activity license by the Medicinal Cannabis Agency for its controlled environment greenhouse cultivation facility based in the Waikato region.
This marks another significant milestone in the Cannasouth group’s progression towards the supply of New Zealand produced, pharmaceutical quality medicinal cannabis into the domestic and international markets.
Cannasouth CEO Mark Lucas says:
“Our cultivation facility is a state-of-the-art sealed hybrid greenhouse, one of only a handful of this design globally.”
“This facility will produce the highest pharmaceutical quality medicinal cannabis flower, and by harnessing the power of the sun we will achieve this in a much more sustainable and cost-effective way than traditional indoor facilities can.
“As cannabis medicines are plant-based, patients are interested in the origin of the products, unlike traditional pharmaceuticals. Large sophisticated medicinal cannabis markets such as Germany are increasingly looking at the provenance and environmental credentials of suppliers and this facility will enable Cannasouth to target these markets, be competitive, and with a great story.”
Now licensed, the cultivation facility will focus on the equipment commissioning, system validation, and GMP quality certification process.
Commercial cultivation operations are scheduled to commence in mid-2021.







